Clinical Manufacturing
Qualify your HCP ELISA to meet and exceed regulatory expectations
To monitor the effectiveness and consistency of downstream purification processes, biomanufacturers rely on HCP ELISAs. But even ELISAs have analytical limitations, necessitating orthogonal confirmation of their ability to detect HCPs. It is critical to ensure that the selected HCP ELISA method is fit for its intended use.
To qualify an ELISA assay, biomanufacturers must show good dilutional linearity, accuracy, precision, and assay range. HCP ELISAs also require an HCP antibody coverage assessment to demonstrate broad reactivity to an array of HCPs in a manufacturing process. Regulatory agencies require biomanufacturers to use orthogonal methods to confirm antibody coverage to individual process-specific HCPs to support further use of a particular HCP immunoassay. A well-developed and qualified HCP ELISA will ensure that HCPs have been reduced to safe levels and that the purification process is consistent from batch to batch.

At Cygnus Technologies, tracking HCPs is more than just a box-checking exercise.
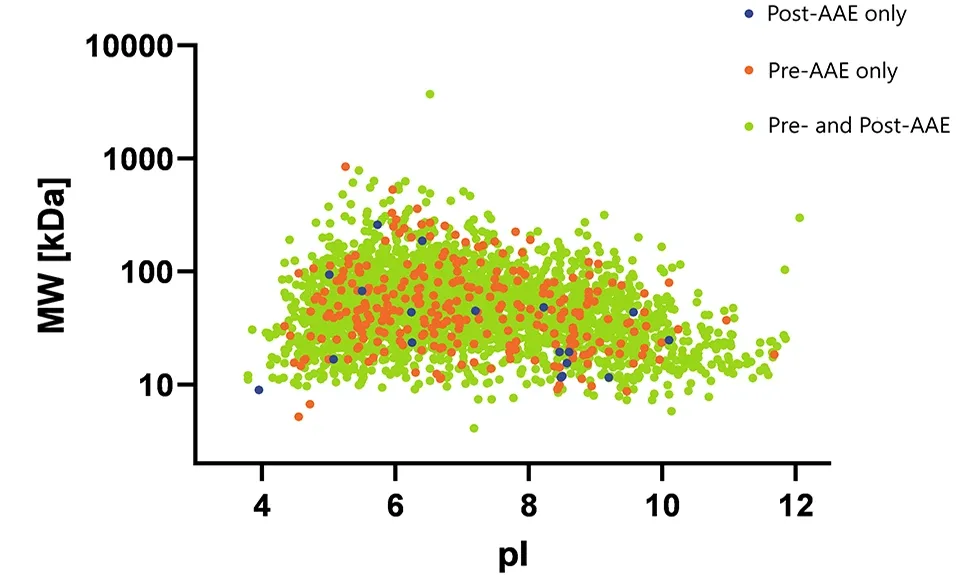
We know firsthand that there are technical challenges and limitations of gel-based orthogonal methods, such as 2D Western blot (2D WB) and 2D differential in blot electrophoresis (2D-DIBE), which used to be the standard for assessing antibody coverage of HCPs. That’s why Cygnus Technologies developed a more sensitive and specific Antibody Affinity Extraction (AAE™) method.
Cygnus’ AAE™ method is:
Access our decades of technical expertise
Cygnus provides coverage analysis services to customers in a state-of-the-art Chromatography and Mass Spectrometry Laboratory. These services feature our AAE™ method coupled with 2-dimensional polyacrylamide gel electrophoresis (2D-PAGE) or mass spectrometry (MS) for HCP antibody coverage analysis, and AAE-MS™ for HCP identification and quantitation in in-process samples and final drug substances.
Cygnus’ Assay Development Team has decades of experience performing HCP ELISA Qualification services, which includes testing for:
Dilution Linearity
Accuracy
Precision
Assay Range: LOD/LLOQ/ULOQ
The Assay Qualification Report will be a starting point for your follow-on internal assay validation. Qualifications are also known as feasibility studies or pre-validation studies.


